Chemicals, salt and plastics industries
Note: Petrochemicals and the modelling of oil refineries are discussed under 'Lineside Industries - Petroleum and LPG'.
Chemical Companies are discussed at the end of this section.
The chemical industry is not a recent development, the word itself comes from 'The Art (or Science) of Khem', Khem being the Arabic word for Black and Egypt being known as the 'Black Land'. Prior to the nineteenth century most chemicals used were naturally occurring minerals and organic matter from living things. Chemicals are seldom used on their own, they are usually required for some other process. In Britain soap manufacture and textiles were two of the biggest customers for chemical works. Textile manufacture required large quantities of alkalis, acids, soaps (see Margarine & Soap), dyes and 'mordants' (see under Dyestuffs). The demands of the textile industry therefore prompted and largely funded the early development of the chemical industry whilst the railways facilitated the delivery of raw materials and the transportation of finished chemicals. As other industries grew in importance the chemical industry developed to service their needs, for example Associated Octel set up a plant in Cheshire in 1940 to make TEL (tetra ethyl lead) for use as an anti-knock agent in petrol, for which they have used a number of 'demoutable' tanks as well as rather some 'continetal' loooking rail tank wagons (see also Lineside Industries - Petroleum and LPG).
Since the early 20th century there have been a number of firms producing very
Common salt has as many uses in the chemical industry as it does in the kitchen, the salt industry is discussed below however two of the more important chemicals derived from salt are caustic soda and chlorine gas.
Vegetable matter remains a major source of chemicals, from the active ingredient in many pharmaceuticals to the linseed oil used to treat cricket bats. Most useful are the oils recovered from crushing seeds, discussed under Lineside Industries - Industries associated with docks and harbours.
One of the most important sources of chemicals during the period under consideration has been the town gas works (discussed under Lineside Industries - Gas Works Coke and Smokeless Fuels) and this was also a major source of nitrate fertilisers.
The history of the British chemical industry has been one of gradual consolidation and amalgamation. In 1926 the British Dyestuffs Corporation merged with Nobel Industries, United Alkali Co and Brunner Mond & Co to form the giant Imperial Chemical Industries or ICI. Chemicals are a wide ranging field however and there remained a number of smaller firms, mostly producing a small range of specialist products. One of the larger of these is the BOC Group, which produces liquefied gasses, notably carbon dioxide, nitrogen and oxygen.
There is a Museum of the Chemical Industry (Gossage Building, Mersey Road, Widnes, WA8 0DF (Tel: 0151 240 1121) which is open every day except Mondays (or was when I visted it some years ago).
General overview of the chemical industry
There are only a limited number of major sources for chemicals; Coal and wood provide carbon and some interesting oily liquids, various minerals such as saltpetre (imported in considerable quantities), limestone (calcium carbonate) and salt are used in a wide range of processes whilst petroleum oil has become increasingly important particularly since the 1950's.
The chemical industry considers itself divided into two branches, heavy and light. The heavy chemical industry deals with raw materials and primary processing, the quantities involved in the heavy chemical industry tend to favour wagon-load or even train-load consignments. The light side of the industry deals mainly with combining or refining the heavy chemicals to produce end-user material and this naturally tends to favour smaller consignments with wagon-load being common but train loads being rare.
Important chemicals can be divided into three main classes; 'organic', acids and alkalis.
Organic chemicals are those based on carbon, mainly the hydrocarbons found in oil, coal and wood, inorganic chemistry deals with materials not involving carbon most of which have little practical application. Most organic chemicals are derived from living or once living things and to date about three million organic chemicals have been identified.
In 1828 a German scientist (Woehler) found a way of synthesising organic Urea from inorganic ammonium cyanate, proving the substances which had been obtained from living organisms were no different than materials obtained from minerals. This was a major breakthrough and changed the face of industrial chemistry.
Pure carbon is itself an important chemical, charcoal, anthracite coal and coke are all fairly pure forms of carbon with many industrial uses. Lamp black is finely divided carbon (better known as 'soot') made by burning oil or coal gas with a restricted amount of air. Lampblack was used to make inks which did not fade and shoe polish but its most important use after the 1930's was in motor car tyres. Rubber tyres are actually about one third carbon, which makes the rubber much more hard wearing and accounts for the black colour. Lampblack was shipped in cloth bags but being a fine powder it leaked out and stained the surrounding area, railway wagons which had been used to carry the stuff needed extra sweeping out to prepare them for their next cargo. Coke, made from coal or obtained from oil, is pure carbon and as well as being a fuel it has a range of uses in industrial chemistry. Similarly charcoal (made from wood) is almost pure carbon and, up to the 1930s charcoal was an important chemical. The by-products of coke and charcoal production also yielded a range of useful chemicals (see also Coal Tar and Wood Tar Distillers).
In 1564 a deposit of graphite (a form of carbon) was found in the lake district that was so pure it could be sawn into sheets and it was used to make rather crude pencils. This stuff was so valuable it was escorted by armed guards when shipped to London. By the 1780's the Lakes graphite was exhausted so experiments started on alternatives, Staedler the German company took up a patent in 1785 which used graphite mixed with clay and baked to form a 'pencil lead', this is the type of pencil we still use today. Graphite was also imported from Ceylon, however it can be made in bulk by
heating coke in an electric furnace. As well as pencil leads, as a lubricant, as a polish for black metals (stove polish) and for electrodes.
Acids
Acids in their pure form may be solids (such as crystals of citric acid from fruit), liquids (such as sulphuric acid) or even a gas (hydrochloric acid). Bases are compounds which react with acids to produce water and a salt, bases which are soluble in water are called alkalis. Salts are acids in which the hydrogen has been replaced by a metal.
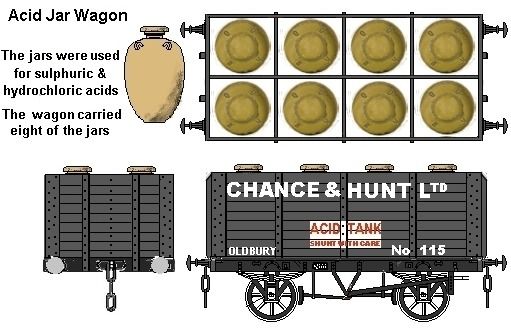
Up to (I believe) the early 1960s a lot of liquid acids were shipped in pottery vessels called 'acid jars', the example shown is typical, it stands nearly six feet high and holds 200 gallons of acid. One common application was the transport of hydrochloric acid (also known as muriatic acid).
Fig ___ An 'acid jar'

These jars would be seen at the works and were transported by rail in specially built wagons (see under hydrochloric acid below).
The mineral acids, sulphuric and nitric, were discovered by the Arabs in about 1000 AD. By far the most important is sulphuric acid which is used in a vast range of processes (including the manufacture of nitric acid) and is probably produced in greater quantities than any other chemical world wide. Consumption per head of sulphuric acid actually gives a fair indication of the industrial capacity of a country. Sulphuric acid or 'vitriol' has been commercially produced in Britain since the 1730's (in the area around Twickenham and Richmond, near London) and by the time the railways were being built the UK was exporting over two thousand tons a year. Oleum is the more concentrated form, sometimes called 'fuming sulphuric acid' (sulphuric acid was originally called 'oil of vitriol' and oleum is the Latin for 'oil').
Sulphuric acid (H2SO4) was in great demand in the expanding industrial economy for making bleach and explosives, allowing the manufacture of Soda instead of expensive potash for soap makers and glass works and freeing more of the available potassium salts to be used for agriculture.
Sulphuric acid was originally produced by heating hydrated iron sulphate (known as 'green vitriol') and absorbing the gas produced in water (hence 'oil of vitriol', a once common term for sulphuric acid). This method was superceeded by burning sulphur in the form of crude sulphur ('brimstone') and nitre (potassium nitrate or 'saltpetre') under a glass bell but in 1746 John Roebuck of Birmingham devised the 'lead chamber process'. This process burned the sulphur and nitre in a furnace, the fumes were then fed into a lead lined chamber with a few inches of water in the bottom. Early lead chambers were modest affairs, a typical works of the early nineteenth century might have six chambers each six foot wide, twelve feet long and about ten feet high with iron trays on which the sulphur and nitre mix was burned. The chambers would be enclosed in a building often of rather crude wooden construction.
In 1827 the Guy-Lussac Tower system was developed, which soon established itself as the most popular method for making the acid. The tower was a wooden affair containing a modified lead chamber and the size increased steadily until by the 1930's they could contain chambers of nearly 30,000 cubic feet (850,000 litres). The base of the chambers inside tower is always supported above the ground so that any leaks will be spotted quickly.
Sulphuric Acid Tower (sketched from a photo from about 1930)
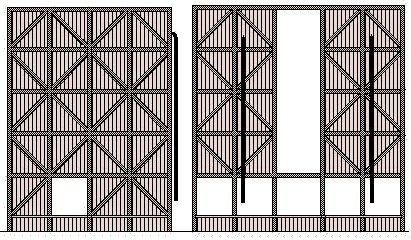
By the 1870's the manufacture of sulphuric acid was well organised and quite efficient whilst cheap saltpetre imported from Chile had brought the cost down. Principal producing areas were London, Lancashire, the North East and Glasgow, although odd plants were in use in or near many cities. In about 1918 an alternative process was developed in which Iron Pyrites (FeS2, a kind of rock sometimes called 'fools gold') was heated to drive off the sulphur. By the 1930s the bulk of the sulphur came from pyrites which we imported from Spain in large quantities. An alternative source of sulphur, widely used to make suphuric acid, was the 'spent oxide' from gasworks (see also Lineside Industries - Gas Works, Coke and Smokeless Fuels).
After the 1880s sulphur was also recovered in a very pure state from the 'alkali waste' of the Leblanc process (discussed below), but this 'recovered sulphur' was too expensive to be burned for making acid. The technology was developed at the Chance Bros establishment in Oldbury near Birmingham (they became Chance & Hunt Limited in the late 1890s and part of ICI in 1926, in April 1999 Chance & Hunt once again became Chance & Hunt Limited, following a management buy-out from ICI and in July 2002 joined the pan-European Azelis group of companies.)
You may also see mention of Copperas Works on old maps, copperas (sometimes called green vitriol) is ferrous sulphate (a compound of iron and sulphur) and can be used to make sulphuric acid by distillation. Copperas occurs naturally and deposits are often associated with coal mining. Copperas works generally disappeared in the second half of the nineteenth century.
In all the above processes the resulting sulphur trioxide (SO3) combines with the water (H2O) to produce the acid (H2SO4). The process released a lot of noxious gasses up the chimneys until the 1860's when legislation was introduced requiring de-nitrifying towers to wash the nitre from the fumes, this could then be recovered and re-used.
1831 Peregrine Phillips developed the contact process for the production of sulphuric acid, it was first used on an industrial scale 1875 but there were several problems to solve and the process was not much used in Britain until about the time of the First World War. After that date it established itself as the standard method of production and continues as such today. The old wooden sulphuric acid towers with their valuable lead lining did not remain standing long after they ceased production and had probably all disappeared by about 1940.
Modern sulphuric acid factories are quite small establishments and feature several small storage tanks similar to those in the Ratio Oil Tank kit, usually silver in colour, and a silver tower with complex external silver pipework roughly two and a half stories high. These towers are very similar to the 'oil refinery' kits available from continental manufacturers, although these would benefit from some additional external pipework for this application and they can be made up using a 'Vicks' inhaler tube with wire pipe-work.
Sulphuric acid can be shipped in glass containers, bottles and carboys, and also in iron tanks, the acid 'passivates' the iron on contact so corrosion does not occur. These tanks could be removable tanks, commonly called 'de-mountable' tanks, and an example of a de-mountable tank for sulphuric acid, a so called 'Vitriol' tank, is available as a kit from Fleetline.
Sulphuric Acid Tank wagon

Carboys are spherical glass bottles about eighteen inches in diameter carried in conical wicker baskets packed with straw (see Volume 1 Cargo & Wagon Loads Fig ___).
Alum (aluminium ammonium sulphate) is a 'mordant', a vital constituent of dyes, and was in great demand for the growing textile industry. Supplies were obtained from mines such as those at Pleasington in Lancashire as early as the seventeenth century. The alum is often found in shale deposits associated with coal mines and a process to recover small amounts of alum from the shale using sulphuric acid was invented in 1850, adding to the demands on the acid manufactures. An alum factory might be quite small, traffic inwards would be shale from a local coal mine and sulphuric acid in glass carboys or iron tanks, outgoing would be XXXX of alum.
Sulphuric acid is such an important chemical there are inevitably concerns regarding supplies of raw materials, with the main problem being pure sulphur. Sulphur is a brittle, pale-yellow, nonmetallic element which has been used since ancient times. Most of the sulphur used comes from deposits in Texas and Chile and although large these are limited.
After the Second World War ICI developed a process for recovering sulphur from a rock called Anhydrite and began commercial mining at Billingham. Anhydrite or calcium sulphate (CaSO4), resembles marble in appearance, it is a hard granular material chemically similar to Gypsum. British rail built special hopper wagons to carry the white granular powder from Billingham to the ICI sulphuric acid plant at Widnes. Sulphur can be recovered from some petroleum oils and by the 1960's something like ten percent of the world supply of sulphur came from oil refineries.
Sulphur is also used to make Calcium Bisulphate (used in paper making to dissolve the unwanted lignin in wood pulp and leave the cellulose fibres), for vulcanising rubber, making matches (the head is actually phosphorous sulphide), as an insecticide and fungicide and in various medicines.
Nitric acid
Nitric acid (HNO3) is difficult stuff to handle as it eats most metals, one of its common names is 'engravers acid'. It is shipped in glass bottles, glass carboys, large non pourous earthenware jars or these days in specially lined railway tank wagons. It used to be made by distilling saltpetre imported from Chile with concentrated sulphuric acid in horizontal cast iron stills, condensing the resulting vapour in stoneware jars called Woulfe's bottles. Someone then came up with vertical earthenware condensers, air was blown up these, condensing the acid vapour (which ran out at the bottom) the remainder being passed to a tall tower where a waterfall dissolved the peroxides and other nasties remaining in the gas. Fuming nitric acid consists of a solution of nitrogen peroxide in concentrated nitric acid and was prepared by distilling dry sodium nitrate with concentrated sulphuric acid.
The big change came when someone worked out how to make the stuff from ammonia (NH3), all you do is heat the ammonia mixed with air with passed a platinum catalyst, producing nitrogen monoxide and steam, this is passed through water (taking more oxygen from the air) to produce the nitric acid. Having said which it took nearly a hundred years to work out the details of this process and it was not used industrially until about the time of the First World War. The early plants were made of stoneware so had to operate at atmospheric pressure, but the development of stainless steels in the mid-1920s enabled pressure operation to be developed. By the early 1930s the heat generated in the process was being used to generate steam to produce power to run the plant, by the 1940s the plants were energy self-sufficient. By the 1960s plants were producing 200 tons per day, by the mid 1970s the figure was about 350 tons per day.
Nitric acid has many uses, most is used to make fertilisers, either for ammonium nitrate or in the various nitrophosphate processes. Ammonium nitrate is also used in the manufacture of explosives and at one time nitrate fertiliser was used by farmers to blow up tree stumps and by terrorists to make bombs but these days it has a small percentage of ammonium sulphate added which renders the nitrate non-explosive.
Up to the 1950's lead nitrate made by treating lead with nitric acid was an important 'mordant' in the textile industry and more recently it has been a regular rail cargo transported in purpose built rail tanks to nuclear reprocessing establishments.
Acetic acid
Acetic acid is an important industrial chemical, in its pure form it is called Glacial acetic acid, most of which is produced in oil refineries by reacting methanol with carbon monoxide in the presence of a catalyst. Acetic acid is used in a range of processes including plastics manufacture, dyes, pesticides, and as a coagulant for latex in rubber manufacture and has been shipped in bulk rail tankers since the late 1960's. It is a clear colourless liquid which does not leave a coloured stain, it is flammable (it ignites at about 800 degrees Celsius) and when shipped it is usually marked as being corrosive Vinegar is acetic acid diluted with water, typically there will be between 4 and 8 percent acetic acid in the mix along with traces of a range of other chemicals. Most vinegar is made by fermenting fruit juice or 'malt' (see under Beer, Ales & Cider) with a mold, the liquid can then be distilled to produce a clear colourless liquid called 'white vinegar'.
Phosphoric acid
Phosphoric acid is another important industrial chemical, it was discovered in the 1850's and has been produced in considerable quantity (it is usually made by combining phosphorous pentoxide with water but usually shipped as a liquid with no water in it called orthophosphoric acid). Phosphoric acid is an important fertiliser (see Fertilisers) and is used in a range of processes including dyeing cotton, sugar refining, soap manufacture, metal pickling & rust proofing, dyes and petrol additives and even as a food additive (notably in 'fizzy' drinks). It is often shipped as a clear liquid in specially lined tank wagons and drums (it attacks iron based metals and eats through glass) but in its pure form it is a solid transparent crystalline material originally shipped in jute bags but these days in lined steel drums. Peco offer a tank wagon in Albright & Wilson livery lettered for carrying phosphoric acid. Note that phosphoric acid tanks are usually heavily stained with white in service.
Hydrochloric acid
Hydrochloric acid (also known as muriatic acid), the third of the 'mineral acids' is actually hydrogen chloride gas (HCl) dissolved in water and its manufacture has until the late 20th century been inextricably related to alkali manufacture as it was a by-product of the Leblanc soda process (discussed below). Hydrochloric acid was once used for making chlorine by adding it to manganese dioxide (in the form of managese ore or recovered from other processes), as the acid eats most metals it was all done in stoneware apparatus until the 1880s when the Weldon process was introduced, employing a complex series of chemical reactions and featuring a tall iron cylinder, say 9 feet (3m) wide and 30 feet (10m) high, called the oxidizer. This recycled the manganeze but required regular shipments of grounf chalk or limestone as well as lime.
These days the situation is reversed, hydrochloric acis is made by mixing sulphuric acid with sodium chloride, itself derived from sodium chlorate (common table salt), the resulting gas dissolves in water to produce the acid.
The sketch below is a 15 ton 9-plank wagon built to carry non-porous earthenware jars of hydrochloric acid, this example was sketched from a photo dated about 1910 I believe.
Fig ___ An 'acid jar' wagon
Both hydrochloric acid and a solid called 'salt cake' (sodium sulphate with impurities) are produced from salt in a device called a Mannheim Furnace, which is a brick lined chamber about ten to fifteen foot in diameter with an iron pan built into the bottom. The furnace is fed through the top and produces hydrochloric acid gas which is piped off and the salt cake which is periodically removed through a door in the side.
Hydrochloric acid oxidised with manganese dioxide produces chlorine, which when mixed with lime produced the 'bleaching powder' (discussed separately below) used in the paper and cloth industries. Hydrochloric acid is used today to prepare metals for galvanising (coating with zinc) or electro-plating (it removes rust from iron for example, the practice is called 'pickling'). It is also used in making photographic materials, drugs and dyes. It is used to make glucose from starch and glue from animal bones and it is important for the plastics industry. It eats metal but can be shipped in rubber lined metal tanks and drums or in glass bottles and carboys.
Salt cake is chemically sodium sulphate (Na2SO4) forms white crystals or powder, it is used in soap manufacture, paper pulping (especially for kraft paper, see Paper & Paper Products), as a filler in synthetic detergents and in processing textile fibres. It has several uses in the manufacture of ceramics and is important for the manufacture of plate glass but since the 1960's it has increasingly been recovered from natural sources. It is shipped either in bags or in metal drums.
Alkalis
The most important alkali has always been Sodium Carbonate, commonly called Soda. Much used in a range of industrial processes relating to everything from glass to soap the original source was burnt seaweed. From the early nineteenth century to around the time of the First World War soda was made by the (French) Leblanc process (Leblanc himself received no remuneration as the revolutionary French government confiscated the valuable patent rights). In Leblanc's process salt is treated with pure sulphuric acid to produce sodium sulphate, this is then burned in a soda furnace with lime and coal to produce crude soda commonly called 'soda ash'. Production started in Liverpool in the early 1820's and plants were established at St Helens soon after.
Fig ___ Typical LeBlanc works

This image is in the public domain
Crude soda ash is a greyish white powder and has many uses in industry. The railways have supplied rolling stock reserved for this traffic since at least the 1920's. One of its uses is in petrol refining and BR reserved some of their COVHOP wagons and the open sand wagons fitted with a sheet supporter specifically for this traffic.
To obtain pure soda the ash was immersed in iron tanks full of water for several hours and the resulting liquor was then evaporated in heated lead pans. Soda is an important chemical in textile production and is used in glass and paper making, aluminium production and in soaps.
The main drawback with Leblanc's process was that it produced fumes which turned into clouds of hydrochloric acid when they mixed with water in the air. Obviously this was a problem which caused considerable litigation, some soda factories had chimneys up to 300 foot high to try and reduce the local damage (that is about two feet high in N gauge). A businessman and engineer called Gossage had interests in the chemical and soap industries and in 1836 he devised a washing tower to remove the dangerous fumes but it was the mid 1860's before these towers were required by law. Once the resulting hydrochloric acid became a useful industrial chemical the acid from these towers was recovered and decanted into large earthenware jars (see picture under acids above). Other materials produce at a larger Leblanc soda works might include; chlorine (which could be used on site to manufacture bleaching powder (discussed in detail below) and other chlorates). Ordinary alkali can be made from the sulphate of soda, also produced were caustic soda and soda crystals. It was common practice to recover sulphur from alkali waste.
At about the turn of the century the British chemists John Brunner and Ludwig Mond developed the alternative ammonia-soda 'Solvay' Soda process and founded Brunner Mond to exploit it. This process was invented in 1861 by Ernest Solvay (1838-1922) and uses salt, ammonia and limestone. A by-product is moderately valuable calcium chloride but it was only after the First World War that this process finally replaced the old salt and sulphuric acid based production. The drawbacks are the amount of energy required (there is a lot of heating and drying in the process) and the chloride effluent that contaminates streams and rivers. Today the high energy cost and more stringent pollution controls have told against the Solvay process and there has been increasing use of naturally occurring deposits of soda, mainly from America.
Caustic Soda
Caustic Soda (sodium hydroxide, sometimes called Lye) was produced as a by-product of the Leblanc soda process and after that from salt water by electrolysis. It is used in a very wide range of processes, one of the more important being the manufacture of soap. Cotton drawn through this stuff under tension comes out stronger and better able to take a dye, this is known as 'mercerisation' and the textile industry also uses caustic soda as a bleaching agent. Caustic soda is used for removing the hair from hides in the leather trade, in paper manufacture it is used to break down wood pulp, oil refineries use it in large quantities as a neutralising agent, it is used to commercially peel fruits and it is the basis of the plastics Rayon and cellophane.
Caustic soda was and is commonly supplied mixed with water and delivered in tank wagons to larger users, I believe some of these tank wagons were also of the 'hutched' type mentioned above. It is also supplied as white pellets in 10 lb cans.
Chlorine
Chlorine, named for its green colour by Sir Humphrey Davey in 1810, is an extremely dangerous substance (used as a poison gas in the First World War). Chlorine gas was originally made from hydrochloric acid but today it is produced from salt by electrolysis
Chlorine was sometimes shipped out pressurised to a liquid in unusual 'hutched tank wagons'. These were conventional cylindrical tanks mounted inside a wooden wagon body with a peaked roof top similar to the salt and lime wagons. There was a small ventilated box on the top to give access to the loading hatch on top of the tank.
Both ICI (Runcorn) and Castner Kellner Alkali (Runcorn)
operated similar tanks for the carriage of liquid chlorine in the 1930's. I am
not sure when they were phased out (or what the function of the external wooden
body was) but I believe some were built with a ten foot wheelbase, suggesting
they were in use in the 1950's. Modelling this wagon is discussed fully in the
section on Kit Bashing.
Fig___ Model and sketch of a hutched tank
wagon
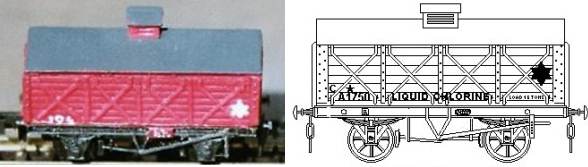
These wagons would be painted light stone with a red band all the way round the body as they would be class A tanks, probably with fast traffic stars and clearly marked Liquid Chlorine on the side.
More recently the gas has been shipped in standard pressurised tank wagons painted white with an orange band to indicate they contain pressurised gas.
Fig ___ Chlorine tank wagons
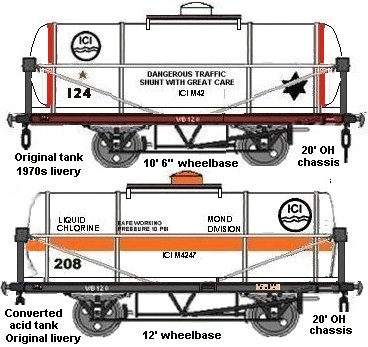
Chlorine forms the basis of a wide range of useful chemicals, 'chlorine bleaches' such as Domestos are actually based on a salt called sodium hypochlorite. Chlorine was used to make 'bleaching powder' (discussed below) and is also used for bleaching wood pulp in paper manufacture and as a disinfectant.
Chlorine was also used to extract bromine from sea water, although you only get a couple of ounces of bromine from a ton or so of water this was worth having as it formed a vital part of the old lead-based anti-knock agent in petrol. At one time there were large factories engaged in this work, mainly in America.
Bleaching Powder
In the late 1700's Charles Tennant, a waever by trade, invented bleaching powder by passing chlorine gas over slaked lime. This seemingly insignificant discovery revolutionised the linen trade by substantially reducing the time, effort and cost involved in the bleaching of cloth prior to dyeing and a huge chemical business was spawned centred on St Rollux, a suburb of Glasgow. Bleaching powder was valuable stuff, commercial production began as the textile industry developed in the 1790's and it sold for over a hundred and forty pounds a ton. The cloth was dipped in a solution of the powder then in a bath of mild acid which released the chlorine itself, the bleached cloth was then dipped in a solution of 'antichlor' and then washed with water. The white powder was shipped in wooden barrels, themselves bleached to a light colour, with rust coloured metal hoops.
A bleach works would typically employ a Weldon still (with its tall metal tower, see under Chlorine above) to produce chlorine gas. There might be a lime kiln on site or lime might be brought in read-made (the lime used was slaked but not wet, so hutched wagons would be used). The lime is spread a few inches deep on trays in lead lined chambers, The chlorine gas admitted to the chamber, where it is absorbed by the lime (it has to be left for about 12 hours for all the chlorine to be absorbed). The powder is then turned over in the trays and the gas treatment repeated, on occasion a third treatment is required to produce the required strength of product.
Not all chlorine is the same, that produced by the Deacon process (a complicated system employing a catalyst) was weak and had to be further dried before it could be used to make bleaching powder. This was done in a tall, usually rectangular, brick tower filled with coke and supplied with a stream of moderately concentrated sulphuric acid. Normal bleaching chambers were impractical with Deacon process gas and very large stone build chambers were built, these were expensive and hard to keep gas tight and by about 1900 were replaced by a different system. This had a series of four horizontal iron tubes, mounted one above the other and each containing an archemedian screw. The powder was fed in at the top, the gas (mixed with air) at the bottom, the gas was forced up through the stack whilst the powder was carried along each pipe in turn by the screw and dropped down to the next level at the end. If the system was working properly the bleaching powder was delivered as a steady stream at the bottom whilst the gas escaping at the top had all chlorine removed and was pure air.
The finished product, a white powdery substance, was usually sold in tierces, that is, casks (barrels) containing 42 gallons (a third of a 'pipe') about four feet high and lined with brown paper. Filling the barrels had to be one of the worst jobs in history, it helped if the casks were on the ground floor and the packers worked through traps in the floor above. The casks had to be kept dry, and preferably cool, and were never exposed to direct sunlight (which could decompose the material and cause an explosion). It was shipped to paper makers and cotton bleachers with smaller quantities supplied to make disinfectants and the like.
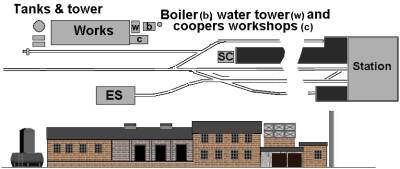
Most bleach works appear to have featured a number of long low buildings aranged around a yard and a tall chimney. Remember the barrels would not be stacked in the open but would be protected from direct sunlight by a canopy on the loading bay. The example shown below is based on the popular Ashburton track plan, this industry gives the place a less rural feel than the traditional mill. Although the 'kick back' siding feeding the 'works' in notoriously troublesome to shunt it is long enough to accomodate the kind of building associated with a bleach works. The sketch includes the tower used in the Weldon still and having the coopers workshops at the front allows for some interesting detailing work.
Fig ___ Track plan including a bleach works
A more concentrated form, based on calcium hypochlorite, has since been developed. Based on different production processes, bleaching powder concentrate also contains calcium chloride or sodium chloride and calcium hydroxide. The effective chlorine content is more than 60%. By contrast, the effective chlorine content in conventional bleaching powder is around 30%.
Acetylene
One of the more important organic chemicals is acetylene gas, which is produced by adding calcium carbide to water. This gas burns well and was widely used for portable lighting such as bicycle lamps as well as for welding and cutting using cylinder-fed oxy-acetylene torches (invented in 1900 by Edmund Fouche - see also Lineside Industries - Industrial and agricultural vehicles and equipment).
The development of electric furnaces in the 1870's enabled the production of calcium carbide by heating a mixture of limestone and coke to a very high temperature. The carbide is a dark grey to black solid and has to be kept in sealed containers to keep out of contact with moisture from the air. It is therefore shipped in a light oil in drums or cased glass bottles. This cheap carbide reduced the cost of acetylene dramatically, but shipping carbide is somewhat problematic as it will react with water in the air to give off the acetylene.
Acetylene gas is itself unstable, if compressed into a cylinder it is likely to spontaneously explode. To get round this problem the acetylene cylinder is filled with a porous material soaked in acetone. The pressurised acetylene dissolves in the acetone and when the cylinder is opened and the pressure drops it bubbles out in the same way carbon dioxide comes out of a 'fizzy' drink when the bottle is opened. Dissolved in the acetone the acetylene is stable and it was removed from the list of official explosives in about 1905, so the cylinders could thereafter be shipped in ordinary railway wagons and vans.
Acetylene is most commonly associated with oxy-acetylene cutting torches but it also forms the basis of a wide range processes and chemicals. One of the more important is 'vinyl chloride' (tetrachloroetane), which is made using acetylene and hydrochloric acid. Vinyl chloride is used to make PVC and forms the basis of a great many widely used plastics (most of the hard plastics used in motor car interiors are based on vinyl chloride).
Ammonia
Ammonia is probably the most important inorganic chemical, it is a gas and by volume the third most important chemical produced today. Ammonia is unpleasant stuff, it combines with water to form corrosive ammonium hydroxide, as there is a lot of water on human skin this makes it dangerous. It is usually shipped as 'anhydrous ammonia' (NH3) refrigerated to -33 degrees centigrade and/or compressed at 125 psi into liquid form.
Ammonia was first recovered from coal gas in about 1850 but at about the time of the first World War the German Fritz Haber (1868-1934) invented a process for synthesizing ammonia using the nitrogen in the air. In the Haber process nitrogen and hydrogen are passed over a catalyst of iron and molybdenum at high temperatures. This was a strategically important development at the time as the ammonia was required for making nitrate type explosives.
Since the 1960s ammonia (NH3) has been mainly obtained from oil refineries from partial combustion of natural gas (mainly methane, CH4). Ammonia was usually shipped dissolved in water (Ammonium Hydroxide) in drums or tank wagons. Since the 1960's pure ammonia gas has been shipped in pressurised tank wagons. These are painted white with an orange band round them and with AMMONIA written on the side in lettering about eight inches to a foot high.
Fig ___ 90 ton ammonia tank wagon

Ammonia is an important fertiliser, either on its own or more commonly in the form of compounds such as ammonium nitrate, it is also used for making both nitric acid and Soda (sodium carbonate) and it has become increasingly important in the manufacture of plastics. Ammonia is used as a 'feed stock' in petro-chemicals, in its pure form it is also used in some types of refrigerator. The salts of ammonia are important for making fertilisers, drugs, dyes and explosives.
Salt
When Sir Humphrey Davy first separated salt into its constituent parts of sodium and chlorine in 1807 no one had any use for either. Today salt is one of the more important chemicals in industry, chlorine (discussed above) is used to make hydrochloric acid, sodium hypochlorite and forms part of several important chemicals (notably PVC or poly vynil chloride). Sodium is used to make various compounds such as sodium carbonate (soda), sodium sulphate, baking soda, sodium phosphate, and sodium hydroxide. Salt is widely used as a preservative for meats and is employed in some refrigeration processes, in dyeing, and in the manufacture of soap and glass. Salt crystals (transparent to infra red) are used as lenses for some laboratory imaging systems.
In Britain today (mid 1980s) there are two major salt producers, ICI and British Salt, both base their salt production on the Cheshire plain. The Romans first exploited the salt deposits of Cheshire on a commercial basis, the town of Middlewich was called 'Salinae' which actually means 'salt works' and all the towns who's names end in 'wich' are connected with the salt industry. Although Cheshire is by far the largest supplier of salt there are other sources in the UK, the Salt Union (see below) operated mines near in Staffordshire and at Durham and Broomsgrove Worcestershire (for which there is a wagon illustrated in Bill Hudson's book Private Owner Wagons Volume 4).
The history of the firms involved in salt production starts in the middle of the 19th century, when demand was booming and a great many small firms set up. This lead to the usual problems of over capacity and fierce competition as the growth in demand began to tail off towards the end of the century.
In 1888 some 64 of these firms (accounting for some 90 percent of the UK total salt production) banded together to form the Salt Union, which acted as a regulatory body somewhat similar to OPEC. The Salt Union funded research and development such as the vacuum drying process mentioned above.
In 1937 the Salt Union was acquired by ICI, who then became the UK's major supplier of salt. After the second world war many of the remaining firms merged, two of the largest being Cerebos (who own Saxa) and Stavely Industries, this latter firm having been involved with salt since 1923 when their British Soda subsidiary built a manufacturing plant at Sandbach.
The joint company built a new plant at Middlewich, next to the Cerebos packing plant and became 'British Salt Ltd' in 1967. Shortly after this Rank Hovis McDougall organisation acquired Cerebos and in 1982 they sold their share of British Salt to Stavely Industries, who then became sole owner.
The Cheshire area is the centre of salt production in the UK, Cheshire itself was served by the LNER, LMS and GWR, all of whom would therefore have taken both salt and its related chemicals traffic.
The methods of salt production remain much the same in principle today as those employed by the Romans; it is 'mined' using water to dissolve the rock, the resulting brine then being dried to produce salt crystals. Originally this drying was done in large drying pans open to the air, but in 1905 the first vacuum evaporation plant was built by the Salt Union at Winsford and this system is now the standard method of refining the material, variations in the process enabling a wide range of grades to be produced.
Various impurities in the salt such as magnesium and calcium are removed by precipitating them in the brine stage using milk of lime, soda ash and boiler flue gas, the resulting waste is these days returned to the brine cavities to prevent subsidence.
Industrial users take salt with about 25% water, food production however requires salt which has been completely dried. Due to the problems of corrosion salt is usually shipped bagged rather than in bulk. Salt was shipped in white cloth bags up to the 1940's, these were about two foot six high by a foot wide and tied off at the top. Each held 40 lbs of salt.
In the early 1980's Cleveland Potash, who mine potash in the North East, began selling rock salt to local authorities for winter road gritting. This material was shipped by rail in XXX wagons and in open topped Cobra containers. One problem with this traffic was that the salt tended to corrode the metal of the wagon or container
Plastics
The modern range of plastics are mainly derived from the products of oil and natural gas refineries, by far the most important ingredient is ethylene, which can also be produced from ethyl alcohol. Ethylene is a highly flammable gas shipped as a liquid, either compressed to very high pressures in steel cylinders or refrigerated down to -104 degrees centigrade in specialised ships. For bulk movement on land it can be partly refrigerated to keep the pressure within sensible limits and shipped in insulated pressure tanks. It forms the basis of most plastic solvents as well as most plastics in common use.
Plastics are not all that new, the term 'polymer' first appeared in 1866. Naturally occurring plastics such as 'Casein' from cows milk and 'zein' which is a protein recovered from corn gluten meal using alcohol, have been used for many years. Casein is made by treating skimmed milk with Rennet enzyme, the resulting precipitate is dried and forms a white powder. Mixed with water this powder tuns into a thermosetting plastic 'dough'. It can be moulded and was used for a range of applications but its main use was as a wood glue. Casein was used to make white 'milk buttons', popular for ladies clothing, although these tended to fail when they got wet.
'Celluloid', a trade name for one of the less explosive varieties of nitro-cellulose (otherwise known as gun cotton), was invented by Alexander Parkes in 1855 and an improved form was first produced commercially by John H. Hyatt in 1872. It is made by the action of nitric and sulphuric acids on cellulose (in the form of wood pulp or cotton), and was commonly used for the separate stiff collars and cuffs on gentleman's shirts.
Cellulose acetate is another old plastic, made by reacting cotton scraps or wood pulp with acetic acid mixed with some sulphuric acid. The result is a white solid thermo-plastic material, shipped as a powder or flakes in cartons, drums or multi-wall paper sacks, it is used in lacquers and other protective coatings and since the 1950's for cigarette filters (before this 'tipped' cigarettes used cork for the filter).
Artificial silk or Rayon was first made in France in the 1884 but it was not produced in quantity successfully for many years. Rayon is a cellulose based fibre made from cotton or wood and a practical method of producing it was patented by Arthur Little (1863-1935) in 1902. This artificial silk was introduced into the Lancashire textile mills in the 1920's and re-named rayon in 1924. Terylene was invented by Whinfield and Dickson in 1941 (the Americans call it Dacron) and silicon polymers were developed in about 1945.
Nylon is a generic name for a range of materials, all called nylon but distinguished by numerical suffixes, these were first developed in 1935 but only became a practical product in the 1940's. The name Nylon comes from the fact that the material (actually Nylon 66) was simultaneously discovered in New York and London. British Railways transported large canisters of nylon filament in the 1950's, collecting them from the chemical factory and delivering to the spinners on heavy low-loading trailers pulled by four wheeled tractors. I have not been able to confirm the type of railway wagon used to transport these containers.
Fig ___ Nylon containers
XXX MORE INFO REQD
Bakelite was a common term for plastics up to the 1960's. This is actually a specific hard brittle plastic made by mixing Phenol and formaldehyde and squirting the mix into a mould. It was the first truly synthetic plastic material and was invented in 1907 by a Belgian called Leo Baekeland who was living in the USA at that time. It was used for a range of household and industrial items, often associated with electrical equipment. Later the word became a trade mark owned by the American Union Carbide company and was applied to a range of materials.
Polythene, made from ethylene gas, was discovered and developed by I.C.I. in the 1930's and after the second world war it became an important packaging material first in the form of polythene bags, later as a film glued to a stiff card backing as the first form of clear 'blister packaging'. Also in the 1930's polytetrafluroethene (PTFE) was invented, usually marketed as Teflon this has an exceptionally low coefficient of friction but it's main use is as a sealing tape when joining threaded pipe sections together (when I joined my first ship I was told in all seriousness by a Senior Engineering Officer that PTFE stood for Pipe Tape For Engineers).
Tetrachloroetane (vinyl chloride, produced using acetylene and hydrochloric acid, both gasses) was first made in 1835, this is the stuff from which PVC and most hard plastic are made, although those products took over a hundred years to develop. Up to the early 1980s vinyl chloride was shipped as a white powder (actually polymerised pellets) but problems had shown up with liver diseased in the workers exposed to it and thereafter it was most commonly shipped as 'VCM' or vinyl chloride monomer, a sweet smelling gas, chilled and shipped as a liquid (no one yet knows if this is actually any safer than the solid form).
Companies operating branded chemical carrying rolling stock in the UK
Imperial Chemical Industries (ICI)
By the early 1920s the German chemical firms had merged into a single entity called IG Farben and in America Du Pont was dominating the chemical industry. British firms were worried about this and formed ICI in 1926 to protect their interests. ICI was created by merging four existing companies; The United Alkali Company, Brunner Mond, Nobel Explosives and British Dyestuffs Corporation. ICI were by far the biggest UK chemicals company, producing chemicals, explosives, fertilisers, insecticides, dyestuff's, non-ferrous metals, and paints. The main production plants were:
Billingham and Wilton (on Teesside). Initially this site produced fertilisers but from the mid 1930s it also produced plastics. During World War II it manufactured synthetic ammonia for explosives. The various works in this area were merged in the 1960 to form ICI Heavy Organic Chemicals Division and ICI Agricultural Division.
The works at Blackley, near Manchester, produced dyestuff's. When the company was divided into divisions in the 1960s this became the ICI Dyestuffs Division and at various times it was combined with other specialty chemicals businesses and became ICI Colours and Fine Chemicals and then ICI Specialties.
The site at Runcorn not far from Liverpool but in Cheshire, produced chlorine and caustic soda. In the 1960s it became ICI Mond Division but later became part of the ICI Chemicals and Polymers Division. In 1991 Brunner Mond Holdings Limited was formed by the break-off of the UK and Kenyan soda ash businesses from ICI, recreating Brunner Mond as an independent company (see also Brunner Mond below).
Dutch firm Akzo Nobel (owner of Crown Berger paints) took over ICI in June 2007.
Fig ___ ICI logo

United Alkali Company Limited
United Alkali was a British chemical company formed in 1890 by Charles Tennant. The firm produced soda ash by the Leblanc process, which was sold to the glass, textile, soap, and paper industries. It became part of ICI in 1926. See also under Tennants Consolidated Ltd below.
Brunner Mond
A soda ash company set up in 1873 by John Brunner and Ludwig Mond and based at Winnington near Northwich, Cheshire they produced their first soda ash in 1874 using the then new Solvay ammonia process. In 1924 Brunner Mond acquired the Magadi Soda Company of Kenya and in 1926 Brunner Mond merged with three other British chemical companies to form Imperial Chemical Industries. The famous ICI limestone hoppers were used to supply the Brunner Mond plant in Cheshire, as of 2008 the trains still run but the wagons are modern high capacity hoppers. In 1991 Brunner Mond Holdings Limited was formed by the break-off of the UK and Kenyan soda ash businesses from ICI, recreating Brunner Mond as an independent company. After this time the Brunner Mond branding was applied to the large hopper wagons carrying limestone. Brunner Mond was purchased by the Indian giant Tata Chemicals in 2006.
British Dyestuffs
British Dyestuffs Corporation Ltd was a British company formed in 1919. The British Government was the company's largest shareholder, and had two directors on the board. The organisation was created because, prior to World War One, British industry had relied on Germany for 89 percent of its dyes, and much of the remainder relied on German produced feed stocks. This caused problems during the war and the plan was to address this shortcoming. To start with the Corporation bought out other firms notably Read Holliday and Sons of Huddersfield. The company supplied a comprehensive range of dyes within a competitive market, its most notable foreign competitors were Du Pont and IG Farben. It became part of ICI in 1926.
Fig ___ British Dyestuffs Corporation logo

Nobel Industries Limited
Nobel Industries was founded in 1870 by Alfred Nobel to produce and market his new explosive 'dynamite'. The factory was set up at Ardeer, on the coast at Ayrshire. The firm went on to produce blasting gelatine, gelignite, ballistite, guncotton, and cordite. After the formation of ICI in 1926 this company became the ICI Nobel Division, after the de-merger of ICI this company was purchased by Inabata & Company (a Japanese trading firm).
Chance and Hunt
A chemical company based in Oldbury they produced a range of chemical products including soda ash and sulfuric and hydrochloric acid. They were established in the early 19th century as a branch of Chance Bros the glass makers of Smethwick to make chemical for the glass works. They expanded their range of chemical to include sulphuric acid (by the chamber process), saltcake, hydrochloric acid and soda ash (described above) and by the 1950s they were also selling sal ammoniac and ammonium carbonate.
In the 1870s they worked out how to recover the sulphur from the waste of the Le Blanc soda ash process and in the 1890s the name changed from Chance Bros to The Oldbury Alkali Co Ltd. and then to Chance and Hunt. They made TNT during World War One but the firm was controlled by Brunner Mond by the end of the war.
Fig ___ A Chance and Hunt 'acid jar' wagon

When ICI was formed Chance and Hunt became part of the company but continued to trade under their old name for certain products until 1939 when they became the Chance and Hunt Department of ICI Mond Division. In the later 1960s a large part of the Oldbury works was taken up by the new motorway and it became just a distribution depot. In 1999 amidst the de-merger of ICI the management bought the company, which became Chance and Hunt Limited, in 2002 they joined the multi-national Azelis group of companies. As of 2008 they were producing a wide range of chemicals from agricultural fertilisers, through a range of adhesives and resins.
Albright and Wilson
A major British chemical company, ranked second only to ICI in the UK chemical industry (a;though much smaller), it was founded in 1856 as a manufacturer of potassium chlorate and white phosphorus for the match industry. Specialising in the field of phosphorus chemistry it was the world's biggest supplier of phosphates for detergents. They operated large sites initially at Oldbury near Birmingham and subsequently additional plants at Avonmouth near Bristol and on the Mersey near Liverpool.
In 1955 Albright and Wilson took over the Marchon Chemical Company based in Whitehaven, which produced phosphorus-based detergents by the "wet" process. The phosphate detergent business rapidly declined in the later 1970s due to problems it was causing in the environment (the name Marchon ceased to exist when the French company, Rhodia, took over Albright and Wilson in 1999).
This lead to a near collapse of the company which was then rescued by a deal with Tenneco Inc. (an American oil company) in 1971.
White phosphorus continued in production at Oldbury until 1972 when production was moved to Newfoundland. The white phosphorous was shipped back to the UK in bulk tankers, discharging at Portishead in Somerset. Although wholly-owned by Tenneco from 1978, Albright & Wilson retained its identity and management until it was divested by a public offering in 1995, as a part of the break-up of Tenneco. The move of the white phosphorous plant to Canada was beset by management failures and caused a slump in profits and the company was absorbed by the French chemical company Rhodia. Parts of the original Albright and Wilson company are now owned by the Huntsman Corporation.
Peco offer a tank wagon in Albright & Wilson livery lettered for carrying phosphoric acid, this can be used on layouts from the mid 60s (when the tank type was introduced) until 2000 or shortly thereafter (when A&W was bought out by the French firm Rhodia). Note that phosphoric acid tanks are usually heavily stained with white in service. This company used a range of chemicals, including liquid chlorine (supplied to their Oldbury works in tankers, I believe by ICI).
Izal
Izal was a subsidiary of Newton, Chambers and Co (founded in 1789 who operated coal mines and iron works). Izal was a disinfectant business, utilising the products of coal distillation (another branch of the Newton Chambers empire), it was formed some time before the First World War and made extensive use of rail transport (Bachman offer a coal wagon in OO). Shortly before that war Izal built some tank wagons (at least 6). I am unsure what these were for as Izal would obtain the distillates from the adjoining works of their parent company, they may have been for bulk delivery but they may also have been used to bring in additional chemicals for producing their range of antibacterial polishes.
Fig ___ Izal tank

At the time Izal was offering 'Disinfectant Fluids and Powders, Insecticidal Fluids and Powders, Liquid Soaps, Cleansers, Antiseptic Toilet Rolls, etc.'. By the 1960s Izal were listed as 'Specialists in the manufacture of various germicides including Izal, Sanizal, Zalpine toilet rolls'. Newton Chambers and Co were taken over by industrial holding company Central and Sheerwood in 1972.
British Oxygen Company The British Oxygen Company began life in the mid 1880's when two French brothers by the name of Brin developed a new method of obtaining oxygen by heating barium oxide and set up Brin's Oxygen Company. The main use for oxygen at the time was 'lime light' in theatres but the company promoted its use in sugar bleaching, preserving milk, making saccharine, vinegar and linoleum, maturing whiskey and in iron and steel manufacture.
Particularly profitable were fizzy oxygenated drinks.
Early distribution was in cloth 'gas bags' but iron cylinders soon followed. The problem with these was that the cylinder weighted and cost a lot more than the gas it contained which restricted the practical distribution. In the later 1880's Brin's therefore began licensing a number of producers throughout the country and in 1890 they patented a new steel cylinder which soon became the international standard. Brin's also expanded into making fittings for these cylinders.
At about the turn of the century a new method of obtaining oxygen direct from the air was independently developed in Britain, Germany and the United States, as often seems to have happened the German team headed by Dr. Carl von Linde reached the patent office first. By 1906 Brin's had obtained a licence to make oxygen using the new system, they abandoned their now outmoded method and changed their name to British Oxygen Company Limited.
There followed a steady expansion as new uses for oxygen were developed, notably in welding and cutting, and with the coming of War in 1914 BOC production was dramatically ramped up to meet the demands of the armament manufacturers.
In the post war years BOC continued to expand, buying up smaller firms and further developing production of acetylene and 'rare gasses' (argon, krypton, neon, xenon and helium). The latter were mainly used for their inert chemical properties for filling various kinds of light fittings and lamps but also in hospital anaesthetics. In 1920 BOC bought Sparklets, who made small cylinders of carbon dioxide for soda siphons, and in 1930 BOC merged with a South African company with whom they had worked on developing oxy-acetylene cutting and welding equipment.
In 1935 BOC designed the Queen Charlotte's Gas-Air Analgesia Apparatus, and in 1936 they introduced Entonox, a new analgesic gas for use in childbirth and in ambulances. In the same year BOC built the first 'ring main' system for distributing oxygen to hospital beds and theatres. At this time they established a new Medical division and produced what was to become the standard gas-air anaesthetic apparatus for use in hospitals.
All medical gases were distributed in steel cylinders
XXXX Sizes?
In 1936 BOC expanded its interests in welding by buying Quasi-Arc Co, who had developed improved electrical welding electrodes.
The second world war placed demands on the company, the Sparklets subsidiary in particular developed its little gas bottles into a great range of types for everything from inflating life-jackets to releasing ether into aircraft engines to help them start in sub zero temperatures.
The 1950's saw a major competitor arrive in Britain, the American Air Products but this was coupled with a dramatic increase in the demand for steel, which required a lot of oxygen. The old methods of shipping the gas in pressurised tanks could in no way meet the demands of the new furnaces and BOC pioneered the development of on-side oxygen production, supplying oxygen by the ton. Other customers for this 'tonnage oxygen' included Wimpey, who used it for rocket motor testing, and as a fuel for the Thor and Blue Streak missiles.
Nitrogen was originally used in electric light bulbs, also for making Ammonia (Haber Process) and up to the 1940's for making Nitric Acid (Birkeland-Eyde Process). Demand increased with the development of flash or freeze drying and in the 1960's BOC set up a joint venture with Linde AG of Germany.
The 1960's and 70's saw a wide ranging diversification of BOC in spite of a scathing report by the Monopolies & Mergers Commission who claimed they were over charging clients (non of the clients had complained however). They went into fatty acid production, resins and adhesives.
Fig ___ A BOC bogie gas tanker from the 1960s

One major area of interest remained gas welding and cutting and BOC were at the forefront of underwater technology that became so important with the development of the North Sea oil fields. In 1978, after years of negotiation with the American authorities, BOC bought the American firm Airco. This doubled the size of BOC and prompted a name change to BOC Group.
In 2006 BOC was bought by the German Linde AG group, which then became the worlds largest gas producing company.
The British Cyanides Company Limited formed in about 1880 as a joint venture by Albright and Wilson and Chance and Hunt to exploit a new process for extracting gold from low-grade ore. Both companies produced cyanide already and their joint subsidiary was established on land between the two existing firms premises in Oldbury near Birmingham. Their main customer was South Africa, but in 1900 the Boer War broke out this market disappeared overnight. They tried a range of products including ferro-cyanide (from sulpho-cyanide using purssiate of soda) . In 1904 they came up with a better method of making cyanide and the company was re-launched and then went into using the barium process to fix nitrogen from the air and produced cyanogen, from which they made sodium cyanide. In World War One they made sodium manganate for gas masks and during the war they set up a potash plant (used to make high quality glass for the Admiralty) and after the war they also made prussate of soda. They were listed a few years later as making 'Yellow Prussiates of Soda and Potash, Carbonate and Bicarbonate of Potash, Permanganate of Potash'. They tried using sulpho-cyanide to make thiourea for use in silk and tafeta but they went out of fashion in the later 1920s they tried condensing thiourea with formaldehyde to produce water-white resins with which they produced 'moulding powders' (powder mixed with the resin, an early form of plastics). By 1930 they were marketing their resins under the name Beetle Products of Oldbury and during the following decade produced a range of resins with all sorts of uses including adding 'wet strength' to paper towels and as a coating on all manner of goods. During this period the name British Industrial Plastics was commonly used and the firm developed the first practical adhesives for making plywood. These glues allowed the building of the Mosquito aircraft in World War Two as well as gliders and small power craft for the Royal Navy. After the war they continued to grow as British Industrial Products but in 1961 they were purchased by Turner and Newall (who made goods for the building industry).
BIP Chemicals Ltd, Oldbury.
See also British Cyanides Company Limited above. BIP (Oldbury) Limited was set up in 1894 and has become a world leader in the supply of thermoset plastics and chemicals with manufacturing and distributors now located worldwide. The company now forms part of the Tennants Consolidated Ltd group. BIP were the first company to patent urea-formaldehyde resins during 1924, since which BIP has evolved into a technology innovator delivering class leading thermoset moulding materials, amino and etherification chemistry, polyurethanes and many industrial chemicals. In 1993 the company restructured to form two companies to reflect the different aspects of its business. BIP Plastics Ltd comprised the three moulding material businesses of Amino Moulding Powders, Polyester Moulding Compounds and Engineering Thermo- plastics. BIP Speciality Resins dealt with the whole of the resins business.
I do not know of any branded rolling stock but given their involvement in urea-formaldehyde resins this site, which is rail connected, would be a likely destination for the BR operated demountable tanks.
Tennants Consolidated Ltd
Tennants can trace its origins back to the late 1700's when the founder Charles Tennant, the son of a farmer and apprenticed as a Weaver invented bleaching powder ((chloride of lime) by passing chlorine gas over slaked lime to form bleaching powder, a mixture comprised of calcium hypochlorite and other derivatives. This seemingly insignificant discovery revolutionised the linen trade by substantially reducing the time, effort and cost involved in the bleaching of cloth prior to dyeing. Tennant Consolidated Limited was established in 1797 and a huge chemical business was spawned centred on St Rollux, a suburb of Glasgow. Tennant had already worked with the chemist Charles Macintosh and helped establish Scotland's first alum works at Hurlet, Renfrewshire. The chemical business founded by Tennant became known as the United Alkali Company Ltd. and eventually merged with others in 1926 to form the chemical giant Imperial Chemical Industries. The chemical works at Springburn closed in 1964. The company still exists and has headquarters in Bath Street and a factory in Maryhill.
Ronuk
A manufacturer of floor polishes and wood treatments based in Brighton, I am not sure when they set up but they were definitely up and running in 1907. They produced a range of polishes and waxes (highly regarded in hospitals for their germicidal properties) and developed the Ronseal range and Colron wood dyes in the 1950s. Ronuk operated several railway tanks and models in their pleasingly different livery have appeared from time to time. I believe the tank pre-dates Ronseal, however there are two main types of polish (liquid and wax) and there are also solvents (mainly turpentine, see also 'Lineside Industries - Coal Tar and Wood Tar Distillers') involved in some polishes. The tank would not be for the turps (that is a Class A liquid and has to have the standard Class A livery), but it may have been used to ship bulk liquid wax to a bottling plant or for brings some chemical to the polish works.
Fig ___ RONUK tank

Ronuk was purchased by Izal Ltd in the late 1960s (see Izal above). Ronuk then became a separate sales division of Izal in 1970 (trading as Roncraft), and was bought three years later by the Sterling Drug Company.
In 1989, Sterling was bought by Eastman Kodak until the multinational photographic company sold all of its do-it-yourself business to Forstmann Little & Co. in 1994. The New York-based investment bank renamed the company to Ronseal Ltd and sold it to Sherwin-Williams in 1997. Ronseal is based at Thorncliffe Park in Sheffield in the UK and also has a thriving business in Dublin, Republic of Ireland.
^
Go to top of page














